Abstract
Introduction: The revised cytogenetic risk stratification of patients with primary myelofibrosis (PMF) divided patients into 3 prognostic categories, with additional new category of very high risk patients (VHR). This score should enhance traditional classification incorporated in the Dynamic International Prognostic Scoring System-Plus (DIPPS-Plus).
Objective: To evaluate the prognostic utility of cytogenetic stratifications (DIPSS-Plus and the revised cytogenetic model) in patients with PMF referred to our institution between 1984 and 2016.
Methods: We retrospectively reviewed the charts of 883 patients with PMF with available cytogenetic analysis at the time of referral to our institution (> 10 metaphases). Cytogenetic was reported according to the International System for Human Cytogenetic Nomenclature. Patients were classified into cytogenetic risks based on DIPSS-Plus (Gangat, JCO, 2011), and the revised cytogenetic model (Tefferi, Leukemia, 2017). Overall survival (OS) was estimated using the Kaplan-Meier method, and groups were compared by the log rank test. Impact of cytogenetic abnormalities on OS was also evaluated by comparing them against patients with diploid karyotype using stepwise Cox regression.
Results: Median age was 66 years (range, 27-88), and 64% of patients were male. The distribution of DIPSS scores was as follows: 8% low, 48% intermediate 1, 44% intermediate 2 and 14% high. OS in each DIPSS category was 53, 46, 26, 15 months (p<0.001). The JAK2, MPL and CALR mutation was present in 55% (n=486), 6% (n=50), and 7% (n=64). Overall, 563 (64%) patients had diploid karyotype. The most frequent abnormal karyotypes were single 20q- (n=68, 8%), single 13q- (n=40, 4.5%), and ≥3 abnormalities (Abn; complex karyotype, CK, n=52, 6%). Among patients with CK, 27 (52%) pts had VHR Abn.
After a median follow-up of 22.4 months (range, 0.5-251); 708 (80%) of patients died. Eighty five patients (10%) developed acute leukemia, 39% of these patients had CK.
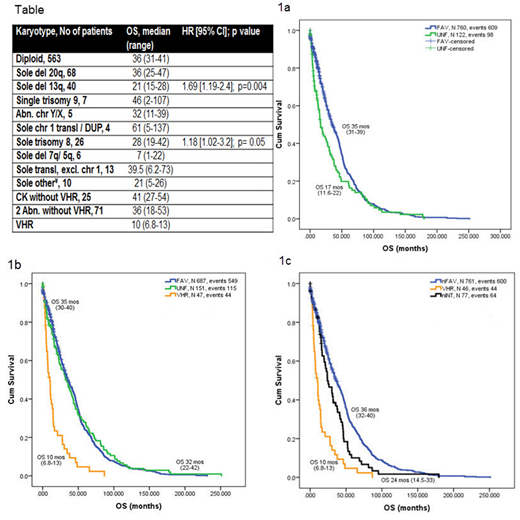
According to DIPSS-plus, patients were stratified into favorable (FAV, n=758, 86%) and unfavorable (UNF, n=126, 14%) category with distinct median OS of 35 months (range, 31-39), and 17 months (range, 11.6-22), p < 0.001 (HR 1.37, [95% CI 1.11-1.7]). Three year OS was 49% and 32%, respectively (Figure 1a).
The revised cytogenetic stratification classified patients into favorable (n=687, 78%), unfavorable (n=151, 17%), and VHR (n=47, 5%) with respective OS of 35, 32 and 10 months (overall p<0.001, FAV vs UNF p= 0.8; Figure 1b); similar between patients in favorable and unfavorable groups. Three year OS for each group was 49%, 46% and 12%, respectively.
OS of patients with individual cytogenetics (as used in the revised classification) is depicted in Table. Patients with single deletion 13q have significantly inferior OS than the remaining patients in FAV group. Patients with sole abnormality of chromosome 1 and trisomy 9 had the longest OS within the FAV group, but without reaching a statistical significance. Similarly, patients with sole trisomy 8, sole deletion 7q/5q, and other sole Abn not included elsewhere, had inferior OS when compared to the remaining patients in UNF group (Table 1). After re-grouping patients with different OS from FAV and UNF groups, we have noticed an intermediate group of patients containing the above mentioned Abn with distinctly different OS from FAV and UNF group of 24 months (range, 14.5-33; Figure 1c).
Conclusions: Results from our cohort of 883 PMF patients did not confirm better discriminatory power of revised cytogenetic stratification model when compared to the DIPSS-Plus, as it failed to differentiate different OS between favorable and unfavorable groups. In our cohort, patients with single deletion 13q, single trisomy 8, and abnormalities of 5q/7q have superior OS to very high risk patients, but inferior to all remaining patients. Because the revised cytogenetic stratification has been already incorporated into newer complex molecular prognostic models of patients with PMF (MIPSSversion2.0, GIPSS), its further validation is warranted.
Table Abbr.: Chr, chromosome, del, deletion, DUP, duplication, transl, translocation, excl, excluding; ¥OTHER solo: INV(9) in [3], Abn chr. 11, 12, 16, 17, 18 (mostly deletion of p/q arms, or addition) [7]; VHR = very high risk (-7; inv(3)/3q21; i(17q); 12p-/12p11.2; 11q-/11q23; autosomal trisomies excl. +8/+9).
Bose:Incyte Corporation: Honoraria, Research Funding; CTI BioPharma: Research Funding; Astellas Pharmaceuticals: Research Funding; Constellation Pharmaceuticals: Research Funding; Pfizer, Inc.: Research Funding; Celgene Corporation: Honoraria, Research Funding; Blueprint Medicines Corporation: Research Funding. Pemmaraju:plexxikon: Research Funding; cellectis: Research Funding; Affymetrix: Research Funding; daiichi sankyo: Research Funding; stemline: Consultancy, Honoraria, Research Funding; novartis: Research Funding; samus: Research Funding; celgene: Consultancy, Honoraria; abbvie: Research Funding; SagerStrong Foundation: Research Funding. Cortes:novartis: Research Funding. Verstovsek:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Italfarmaco: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal